某矿石由前20号元素中的4种组成,其化学式为WYZX。X、Y、Z、W分布在三个周期,原子序数依次增大,W、Y为金属元素,X原子的最外以怪电子数是次外层电子数的3倍,W能与冷水剧烈反应,Y、Z原子的最外层电子数之和与X、W原子的最外层电子数之和相等,Y、Z位于同周期,Z单质是一种良好的半导体。下列说法正确的是
( )
| A. | 原子半径:W>Y>Z>X | B. |
|
| C. | 气态氢化物的稳定性:X<Z | D. |
|
| E. |
最高价氧化物对应水化物的碱性:Y>W
|
F. |
Y、Z的氧化物都有两性
|
面粉厂必须严禁烟火的主要原因是
| A.防止火灾发生 |
| B.防止污染面粉 |
| C.吸烟有害健康 |
| D.面粉颗粒极小,当其扩散在空气中与空气充分接触,一旦引发反应,极易发生爆炸 |
工业生产硫酸过程中的一步反应是2 SO2 (g) + O2 (g)  2 SO3(g)。如果该反应在密闭容器内进行,能说明该反应达到化学平衡状态的是
2 SO3(g)。如果该反应在密闭容器内进行,能说明该反应达到化学平衡状态的是
| A.SO2 完全转化为SO3 |
| B.消耗2 mol SO2的同时生成2 mol SO3 |
| C.SO2、O2与SO3 的物质的量之比为2∶1∶2 |
| D.SO2、O2与SO3的浓度不再随时间变化 |
下列叙述中,不正确的是
| A.汽油是有固定沸点的纯净物 |
| B.煤是由有机物和无机物组成的复杂混合物 |
| C.聚乙烯是通过加聚反应制得的高分子化合物 |
| D.油脂是高级脂肪酸与甘油生成的酯 |
下列关于甲烷、乙烯、苯三种烃的比较中,正确的是
| A.只有甲烷不能因化学反应而使酸性高锰酸钾溶液褪色 |
| B.在空气中分别完全燃烧等质量的这三种烃,苯消耗的氧气最多 |
| C.除甲烷外,其余两种分子内所有原子都共平面 |
| D.甲烷和苯属于饱和烃,乙烯是不饱和烃 |
如图所示,两电极一为碳棒,一为铁片,若电流表的指针发生偏转,且a极上有大量气泡生成,则以下叙述正确的是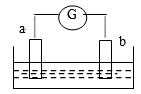
| A.a为负极,是铁片,烧杯中的溶液为硫酸 |
| B.b为负极,是铁片,烧杯中的溶液为硫酸铜溶液 |
| C.a为正极,是碳棒,烧杯中的溶液为硫酸 |
| D.b为正极,是碳棒,烧杯中的溶液为硫酸铜溶液 |