以硼氢化合物NaBH4(B元素的化合价为+3价)和H2O2作原料的燃料电池,负极材料采用Pt/C,正极材料采用MnO2,其工作原理如图所示。下列说法正确的是 
| A.电池放电时Na+从b极区移向a极区 |
| B.每消耗3 mol H2O2,转移的电子为3 mol |
| C.电极a采用MnO2,MnO2既作电极材料又有催化作用 |
| D.该电池的负极反应为:BH4-+8OH--8e-=BO2-+6H2O |
下列物质分别与NaOH的醇溶液共热后,能发生消去反应,且生成物只有一种的是( )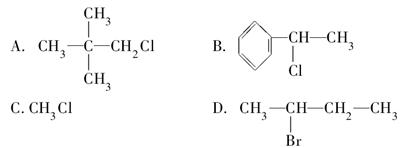
某化合物的结构(键线式)及球棍模型如下: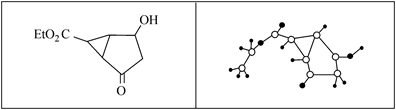 、
、
该有机分子的核磁共振氢谱图如下(单位是ppm):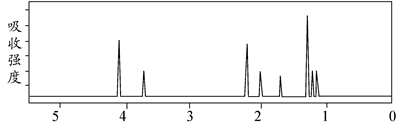
下列关于该有机物的叙述正确的是 ( )
| A.该有机物不同化学环境的氢原子有8种 |
| B.该有机物属于芳香族化合物 |
| C.键线式中的Et代表的基团为—CH3 |
| D.该有机物在一定条件下能够发生加成反应、取代反应,但不能发生消去反应 |
下列有机物的命名正确的是 ( )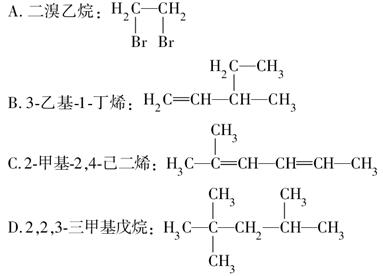
2,2,4三甲基戊烷与氯气发生取代反应时,生成的一氯代物可能有( )
| A.2种 | B.3种 | C.4种 | D.5种 |
下图是一些常见有机物的转化关系,关于反应①~⑦的说法不正确的是( )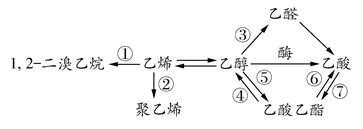
| A.反应①是加成反应 | B.只有反应②是加聚反应 |
| C.只有反应⑦是取代反应 | D.反应④⑤⑥是取代反应 |