常温下,下列溶液中的微粒浓度关系正确的是
| A.新制氯水中加入固体NaOH:c(Na+) = c(Cl-) + c(ClO-) + c(OH‑) |
| B.pH = 8.3的NaHCO3溶液:c(Na+) > c(HCO3-) > c(CO32-) > c(H2CO3) |
| C.pH = 11的氨水与pH = 3的盐酸等体积混合:c(Cl-)=c(NH4+) > c(OH‑) = c(H+) |
| D.0.2mol/LCH3COOH溶液与0.1mol/LNaOH溶液等体积混合:2c(H+) – 2c(OH-) = c(CH3COO-) –c(CH3COOH) |
下列反应的离子方程式书写中,正确的是()
| A.将氨水逐滴滴加到AlCl3溶液中:Al3++3OH-=Al(OH)3↓ |
| B.铜与稀硝酸反应:Cu+4H+ +NO3-= Cu 2++NO↑+2H2O |
| C.硫酸与氢氧化钡在溶液中反应: Ba2++OH-+H++SO42-=BaSO4↓+H2O |
| D.用FeCl3溶液腐蚀铜箔制造的印刷电路板:2Fe3++Cu=2Fe2++Cu2+ |
下列说法不正确的是()
| A.绿色化学的核心就是在生产过程中减少污染 |
| B.形成酸雨的主要物质是硫氧化物和氮氧化物(NOx) |
| C.大气污染物主要来自化石燃料和工业生产过程产生的废气 |
| D.水华、赤潮等水体污染是由于含氮、磷的大量污水任意排放造成的 |
糖类、脂肪和蛋白质是维持人体生命活动所必需的三大营养物质,以下叙述正确的是()
| A.淀粉、纤维素没有甜味,因此不属于糖类 |
| B.油脂属于酯类化合物,油脂的水解反应叫皂化反应 |
| C.葡萄糖能发生氧化反应和水解反应 |
| D.浓硝酸溅在皮肤上,使皮肤呈黄色是由于浓硝酸和蛋白质发生了颜色反应 |
下列化学用语的书写正确的是()
| A.乙酸的分子式:C2H4O2 | B.乙醇的结构简式:C2H6O |
C.F原子结构示意图: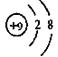 |
D.四氯化碳的电子式: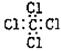 |
可逆反应2NO2(g)  2NO(g) + O2(g),在体积不变的密闭容器中进行,达到平衡状态的标志是()
2NO(g) + O2(g),在体积不变的密闭容器中进行,达到平衡状态的标志是()
①单位时间内生成n molO2的同时生成2n molNO2
②单位时间内生成n molO2的同时生成2n molNO
③NO2、NO、O2的物质的量浓度比值为2∶2∶1 ④混合气体的颜色不再改变
⑤混合气体的密度不再改变 ⑥混合气体的总压强不再改变
| A.①④⑥ | B.②③⑤ | C.①③④ | D.全部 |