25oC时,取浓度均为0.1mol·L-1的醋酸溶液和氨水溶液各20 mL,分别用0.1mol·L-1NaOH溶液、0.1 mol·L-1盐酸进行中和滴定,滴定过程中pH随滴加溶液的体积变化关系如图所示。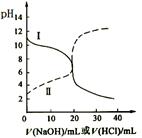
下列说法正确的是
| A.曲线I中滴加溶液到10 mL时:c(CH3COO-)>c(Na+)>c(H+)>c(OH-) |
| B.曲线I中滴加溶液到25 mL时:c(NH4+)>c(C1-)>c(H+)>c(OH-) |
| C.曲线II中滴加溶液在10 mL~25 mL之间存在:c(NH4+)=c(Cl-)>c(OH-)=c(H+) |
| D.曲线II中滴加溶液到10mL时:c(CH3COO-)-c(CH3COOH)=2[c(H+)-c(OH-)] |
一定条件下,溶液的酸碱性对TiO2光催化染料R降解反应的影响如图所示。下列判断正确的是
| A.在0-50min之间, pH ="2" 和 PH=" 7" 时 R 的降解百分率相等 |
| B.溶液酸性越强, R的降解速率越小 |
| C.R的起始浓度越小,降解速率越大 |
| D.在 20-25min之间, pH =" 10" 时 R 的平均降解速率为 0.04mol·L-1·min-1 |
已知: CH3CH2CH2CH3(g)+6.5O2(g) ==4CO2(g)+5H2O(l)ΔH=-2 878 kJ·mol-1
(CH3)2CHCH3(g)+6.5O2(g) ==4CO2(g)+5H2O(l)ΔH=-2 869kJ·mol-1
下列说法正确的是()
| A.正丁烷分子储存的能量大于异丁烷分子 |
| B.正丁烷的稳定性大于异丁烷 |
| C.异丁烷转化为正丁烷的过程是一个放热过程 |
| D.异丁烷分子中的碳氢键比正丁烷的多 |
完全燃烧一定质量的无水乙醇,放出的热量为Q,为完全吸收生成的CO2,并使之生成正盐Na2CO3,消耗掉0.8mol/L NaOH溶液500mL,则燃烧1mol无水酒精放出的热量是
| A.0.2Q | B.0.1Q | C.5Q | D.10Q |
根据碘与氢气反应的热化学方程式
(Ⅰ) H2(g)+I2(g) 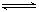 2HI(g)H="-9.48" kJ ▪mol-1
2HI(g)H="-9.48" kJ ▪mol-1
(Ⅱ) H2(g)+I2(s) 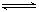 2HI(g)H="+26.48" kJ ▪mol-1
2HI(g)H="+26.48" kJ ▪mol-1
下列判断正确的是()
| A.254 g I2(g)中通入2 g H2(g)反应放热9.48 kJ |
| B.1 mol固态碘与1 mol气态碘所含的能量相差17.00 kJ |
| C.反应(Ⅰ)的产物比反应(Ⅱ)的产物稳定 |
| D.反应(Ⅱ)的反应物总能量比反应(Ⅰ)的反应物总能量低 |
在36 g碳不完全燃烧所得气体中,CO占1/3体积,CO2占2/3体积,且
C(s) + 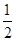 O2(g) = CO(g)△H =" -110.5" kJ/mol CO(g) +
O2(g) = CO(g)△H =" -110.5" kJ/mol CO(g) + 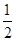 O2(g) = CO2(g)△H =" -283" kJ/mol与这些碳完全燃烧相比,损失的热量是( )
O2(g) = CO2(g)△H =" -283" kJ/mol与这些碳完全燃烧相比,损失的热量是( )
| A.172.5 kJ | B.1149 kJ | C.283kJ | D.517.5 kJ |