工业上制备纯硅反应的热化学方程式如下:SiCl4(g)+2H2(g)  Si(s)+4HCl(g);△H=+QkJ·mol-1(Q>0),某温度、压强下,将一定量的反应物通入密闭容器进行以上的反应(此条件下为可逆反应),下列叙述正确的是( )
Si(s)+4HCl(g);△H=+QkJ·mol-1(Q>0),某温度、压强下,将一定量的反应物通入密闭容器进行以上的反应(此条件下为可逆反应),下列叙述正确的是( )
| A.反应过程中,若增大压强能提高SiCl4的转化率 |
| B.若反应开始时SiCl4为1mol,则达到平衡时,吸收热量为QkJ |
| C.反应至4min时,若HCl的浓度为0.12mol·L-1,则H2的反应速率为0.03mol/(L·min) |
| D.当反应吸收热量为0.025QkJ时,生成的HCl通入100mL1mol·L-1的NaOH恰好反应 |
将足量CO2通入NaOH和Ba(OH)2的混合稀溶液中,生成沉淀的物质的量(n)和通入CO2气体的体积(V)的关系如右图所示,图中AB段表示的离子方程式先后顺序正确的是
①CO2 + OH-→ HCO3-
②CO2 + 2OH- → CO32-+ H2O
③CO32- + Ba2+ →BaCO3↓
④BaCO3 + CO2 + H2O →Ba2+ + 2HCO3-
⑤CO32-+ CO2 + H2O → 2HCO3-
| A.③① | B.②⑤ | C.⑤④ | D.④② |
某化学小组用下图所示装置制取氯气,下列说法不正确的是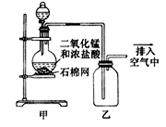
| A.该装置图中至少存在两处明显错误 |
| B.烧瓶中的MnO2可换成KMnO4 |
| C.在乙后连一盛有饱和食盐水的烧杯可进行尾气处理 |
| D.在集气瓶的导管口处放一片湿润的淀粉碘化钾试纸可以证明是否有氯气逸出 |
普拉西坦是一种改善记忆、抗健忘的中枢神经兴奋药,其结构如右图,下列关于普拉西坦的说法正确的是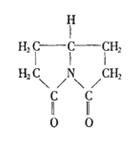
A.它既有酸性,又有碱性
B.它的二氯取代物有两种
C.它可以发生水解反应
D.分子中C、N、O原子均处于同一平面
室温下,有pH=3的盐酸、硫酸、醋酸(假设HAc的电离度为1%)三种相同体积的溶液。以下叙述错误的是
| A.测定其导电性能相同 |
| B.与足量的锌粉反应的起始速率相同 |
| C.与足量的锌粉反应产生氢气的体积比为1∶1∶100 |
| D.与同浓度氢氧化钠溶液反应,消耗氢氧化钠溶液的体积为1∶2∶100 |
在下列条件下,两种气体的分子数一定相等的是
| A.同温度,同体积的N2和O2 | B.同质量,不同密度的N2和CO |
| C.同压强,同体积的H2和CH4 | D.同体积,同密度的CO2和N2 |