下列说法不正确的是
| A.ΔH>0、ΔS>0的反应在任何温度下都不能自发进行 |
| B.反应NH4HCO3(s)===NH3(g)+H2O(g)+CO2(g)ΔH=+185.57 kJ·mol-1 之所以能自发进行,原因是体系有自发地向混乱度增加的方向转变的倾向 |
| C.因为焓变和熵变都与反应的自发性有关,因此焓变或熵变均不能单独作为反应自发性的判据 |
| D.在其他外界条件不变的情况下,使用催化剂,可以改变化学反应进行的速率 |
NM-3和D-58是正处于临床试验阶段的小分子抗癌药物,结构如下: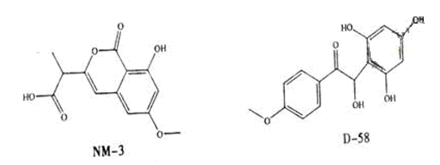
关于NM-3和D-58的叙述,错误的是
| A.都能与NaOH溶液反应,原因不完全相同 |
| B.都能与溴水反应,原因不完全相同 |
| C.都不能发生消去反应,原因相同 |
| D.遇FeCl3溶液都显色,原因相同 |
褪黑素是一种内源性生物钟调节剂,在人体内由食物中的色氨酸转化得到。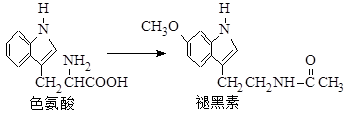
下列说法不正确的是
| A.色氨酸分子中存在氨基和羧基,可形成内盐,具有较高的熔点 |
| B.在色氨酸水溶液中,可通过调节溶液的pH使其形成晶体析出 |
| C.在一定条件下,色氨酸可发生缩聚反应 |
| D.褪黑素与色氨酸结构相似,也具有两性化合物的特性 |
β—紫罗兰酮是存在于玫瑰花、番茄等中的一种天然香料,它经多步反应可合成维生素A1。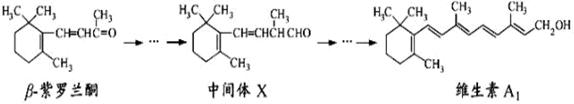
下列说法正确的是
| A.β—紫罗兰酮可使酸性KMnO4溶液褪色 |
| B.1mol中间体X最多能与2molH2发生加成反应 |
| C.维生素A1易溶于NaOH溶液 |
| D.β—紫罗兰酮与中间体X互为同分异构体 |
下列叙述中正确的是
| A.医用酒精的浓度通常为95% |
| B.单质硅是将太阳能转变为电能的常用材料 |
| C.淀粉、纤维素和油脂都属于天然高分子化合物 |
| D.合成纤维和光导纤维都是新型无机非金属材料 |
分析下表中各项的排布规律,按此规律排布第26项应为
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
| C2H4 |
C2H6 |
C2H6O |
C2H4O2 |
C3H6 |
C3H8 |
C3H8O |
C3H6O2 |
C4H8 |
C4H10 |
A.C7H16 B.C7H14O2 C.C8H18 D.C8H18O