在不同温度下,向VL密闭容器中加入0.5 mol NO和0.5 mol活性炭,发生反应:2NO(g)+C(s) N2(g)+CO2(g) △H=—QkJ·mol-1 (Q>0),达到平衡时的数据如下:
N2(g)+CO2(g) △H=—QkJ·mol-1 (Q>0),达到平衡时的数据如下:
| 温度/℃ |
n (C)/mol |
n(CO2)/mol |
| T1 |
|
0. 15 |
| T2 |
0. 375 |
|
下列有关说法正确的是
A.由上述信息可推知:T1>T2
B.T2℃时,若反应达平衡后再缩小容器的体积,c(N2):c(NO)增大
C.T1℃时,若开始时反应物的用量均减小一半,平衡后NO的转化率增大
D. T1℃时,该反应的平衡常数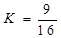
在相同温度和压强下,将等质量的硫分别在足量的纯氧气和空气中燃烧,设前者放出的能量为Q1,后者放出的能量为Q2,则Q1和Q2的相对大小关系为
| A.Q1=Q2 | B.Q1>Q2 | C.Q1<Q2 | D.无法判断 |
下列说法正确的是
| A.ⅠA族元素的金属性比ⅡA族元素的金属性强 |
| B.ⅥA族元素的氢化物中,稳定性最好的其沸点也最高 |
| C.同周期非金属氧化物对应的水化物的酸性从左到右依次增强 |
| D.短周期中次外层电子数是最外层电子数2倍的元素一定是非金属元素 |
2010年诺贝尔化学奖授予理查德·赫克等三位科学家,以表彰他们在“钯催化交叉偶联”方面的研究。下面关于催化剂的说法正确的是
| A.催化剂只改变反应的正反应速率 |
| B.催化剂通过升高反应的活化能来加快反应速率 |
| C.催化剂能够改变反应的反应热 |
| D.催化剂不能改变反应物的转化率 |
欲探究某矿石可能是由FeCO3、SiO2、Al2O3中的一种或几种组成,探究过程如下图所示。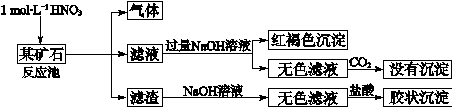
(1)Si在周期表中的位置是。
(2)下列说法正确的是。
a.酸性:H2CO3>H2SiO3b.结合质子的能力:CO32->AlO2->HCO3-
c.稳定性:H2O>CH4>SiH4 d.离子半径:O2-<Al3+
(3)该矿石的组成是,滤渣和NaOH溶液反应的离子方程式是。
(4)该矿石和1 molL-1HNO3反应的离子方程式。
(5)工业上依据上述实验原理处理该矿石,将反应池逸出的气体与一定量的O2混合循环通入反应池中,用化学方程式解释该方法的目的:;若按以上方案:NO与O2循环通入反应池处理该矿石2.36103 kg,得到滤渣1.2103 kg,理论上至少需要1 molL-1 HNO3的体积为L。
将质量相等的铁片和铜片插入氯化钠溶液中,铜片与电源的正极相连,铁片与电源的负极相连,以I=1A的恒定电流强度进行电解,下列有关说法正确的是
| A.阳极上产生能够使湿润淀粉碘化钾试纸变蓝的气体 |
| B.电解一段时间后,电解池的温度升高10℃,此时铁片上析出气体的速率加快 |
| C.电解时钠离子向铜片电极方向移动 |
D.电解一段时间后溶液中会出现蓝色沉淀,总反应为:Cu+2H2O Cu(OH)2↓+H2↑ Cu(OH)2↓+H2↑ |